“Switching on” iron in clay minerals†
Abstract
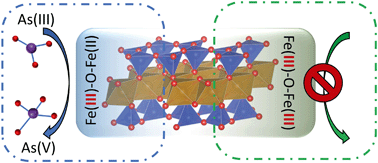
Being the fourth most abundant element in the Earth's crust, iron (Fe) is a key player in myriad biogeochemical processes. Iron that resides in the structures of nano- to micron-scale clay mineral particles undergoes cycling between Fe(II) and Fe(III). This iron comprises a large redox-active pool in surface environments, controlling the fate and transport of nutrients and contaminants. The mechanism of electron transfer involving this iron species is poorly understood. We observe that Fe(III) in clay minerals does not oxidize arsenic As(III), unless a minor amount of Fe(II) is introduced into the predominantly-Fe(III) structure. These “activated” clay minerals are redox-active both in the presence and absence of oxygen. In the presence of oxygen, Fe(II) catalyzes the production of reactive oxygen species; however, the oxidation pathway in the absence of oxygen is unknown. Here we show that under oxygen-free conditions, the redox-active species in clay minerals is FeII–O–FeIII moieties at the edge sites. We used in situ and ex situ spectroscopic methods, including X-ray absorption, Mössbauer, and diffuse reflectance spectroscopies, as well as ab initio calculations. Our ab initio calculations show that desorption of water from an FeII–O–FeIII site in clay mineral requires less energy, compared to a fully-oxidized FeIII–O–FeIII site. We propose that this lower barrier for the desorption of water increases the apparent kinetics of redox reactions on clay mineral surfaces.
- This article is part of the themed collection: Best Papers 2019 – Environmental Science: Nano


 Please wait while we load your content...
Please wait while we load your content...
