Nanoparticle foam flotation for caesium decontamination using a pH-sensitive surfactant†
Abstract
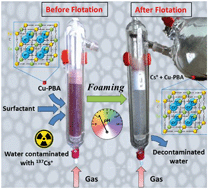
Ion extraction processes show some limitations related to their selectivity, efficiency, cost and ecological footprint. The recent Fukushima event has stimulated the hunt for innovative extraction processes of the caesium ion. Here, we propose a caesium foam flotation process of a highly selective complexing agent, namely the copper based Prussian blue analogue (Cu-PBA), using a pH-sensitive foaming agent (collector): a polyethoxylated amine surfactant (Ethomeen®). The foaming properties, surface charge and surface activity of this complex chemical system have been investigated as a function of pH. It was demonstrated that the pH controls the flotation efficiency through electrostatic interactions between the surfactant and the Cu-PBA. The flotation was highly efficient for pH < 5, where all of the Cu-PBA was extracted by the foam. In contrast, for pH > 10, all of the Cu-BPA remained in the foaming solution and was not extracted in the foam. As a consequence, reversible extraction/de-extraction of the Cu-PBA was made possible through pH cycles, which enables the recycling of the surfactant, reducing both the cost and the environmental impact of the overall process.
- This article is part of the themed collection: Interfaces Against Pollution


 Please wait while we load your content...
Please wait while we load your content...