Ligand directed debromination of tetrabromodiphenyl ether mediated by nickel under visible irradiation†
Abstract
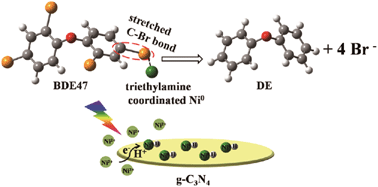
Polybrominated diphenyl ethers (PBDEs) with low bromine substitution, represented by 2,2′,4,4′-tetrabromodiphenyl ether (BDE47), display high biological toxicity. These compounds are highly mobile in aquatic environments, yet their reductive conversion is challenging due to thermodynamic and kinetic considerations. Herein, we develop a catalytic system based on metallic Ni (formed in situ on carbon nitride) that accomplished the complete debromination of BDE47 under visible irradiation with triethylamine as the activity directing agent and electron/proton donor. Employment of triethylamine enabled a more competitive carbon–bromine bond activation, via either nucleophilic attack by surface hydrogen/hydride species on the Ni surface or oxidative addition to the Ni site, with less interference from hydrogen evolution. Density functional theory (DFT) calculation suggested that surface coordination of TEA promotes the bond weakening of BDE47 on metallic Ni. Such a cost-effective system with a benign hydrogen donor is promising for the elimination of PBDEs and other halogenated aromatic contaminants.


 Please wait while we load your content...
Please wait while we load your content...