Removal and recovery of Pb from wastewater through a reversible phase transformation process between nano-flower-like Mg(OH)2 and soluble Mg(HCO3)2†
Abstract
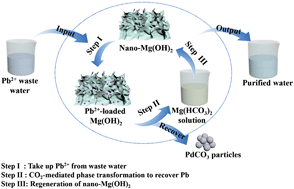
This study investigated the effectiveness of a new technique for recovering lead (Pb) as highly pure PbCO3 nanoparticles from Pb2+-bearing wastewater by using a nano-Mg(OH)2 adsorbent. Microcosmic mechanism studies revealed that the reaction between Pb2+ and nano-Mg(OH)2 was more complicated than previously thought, where a surface defect-induced dissolution and recrystallization process was observed, and Pb-loaded nanowaste with different crystalline lead compounds was produced depending on the Mg : Pb molar ratio. Moreover, the kinetic limits of the reaction of Pb2+ with nano-Mg(OH)2 were found to be much lower than those with common bulk reactants (such as CaCO3 and Ca5(OH)(PO4)3). At Mg : Pb molar ratios >2, the emission standard of Pb2+ wastewater (<0.5 mg L−1) can be met and the treatment time can be greatly shortened. Subsequently, during the carbonation treatment of the Pb-loaded nano-waste, the residual Mg(OH)2 in the nanowaste can be transformed into soluble Mg(HCO3)2, while the coexisting multiple lead compounds can be transformed into insoluble single species of PbCO3 nanoparticles. As a result, more than 99.2% of Pb2+ in the Pb-loaded nano-waste can be recovered. Based on the calcination and hydration treatment, over 90% of Mg2+ in Mg(HCO3)2 solution can be converted to MgO nano-sheets and then regenerated to the nano-Mg(OH)2 adsorbent, which can be re-used to dispose of Pb2+ containing wastewater in the next cycle. The results indicate the potential of the reversible phase transformation approach to recycle heavy metals from wastewater using the nano-Mg(OH)2 adsorbent without generating secondary solid waste.


 Please wait while we load your content...
Please wait while we load your content...