Adsorption of aromatic carboxylic acids on carbon nanotubes: impact of surface functionalization, molecular size and structure†
Abstract
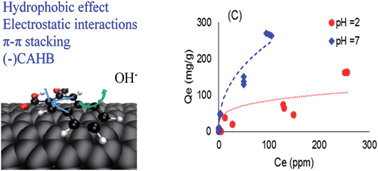
A large quantity of emerging contaminants are ionizable, and the ionized compounds display different adsorption behaviors than their neutral counterparts. In particular, a strong intermolecular force, negative charge assisted hydrogen bonding ((−)CAHB), was recently identified, which explains the unusually strong adsorption of negatively charged compounds on carbon nanotubes with oxygen-containing functional groups. However, most previous studies only probed molecules with one benzene ring. The adsorption of ionizable compounds with more than one benzene ring and additional functional groups has not been examined. This study investigated the effect of surface functionalization, molecular size and structure of six aromatic carboxylic acids on their adsorption on multi-walled carbon nanotubes (MWNTs) in batch reactors. In addition, the short-range interactions of the neutral acids with MWNTs were calculated to evaluate the effect of aromaticity and bulkiness. Hydrophobicity and electrostatic interactions dominate the intermolecular forces between ionized contaminants and MWNT surfaces. pH dependent octanol/water partitioning coefficient (Dow) is a more precise indicator of the adsorption of ionizable compounds on MWNTs. (−)CAHB is a significant force only for compounds with one benzene ring. Hydroxyl and carboxyl functional groups displayed similar capacity to form (−)CAHB, as indicated by the release of hydroxide ions.


 Please wait while we load your content...
Please wait while we load your content...