NO2 and natural organic matter affect both soot aggregation behavior and sorption of S-metolachlor†
Abstract
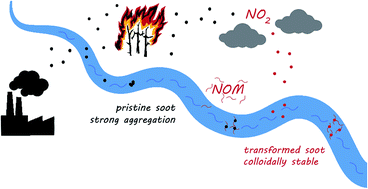
Soot is an important carbonaceous nanoparticle (CNP) frequently found in natural environments. Its entry into surface waters can occur directly via surface runoff or infiltration, as well as via atmospheric deposition. Pristine soot is likely to rapidly undergo aggregation and subsequent sedimentation in aquatic environments. Further, soot can sorb a variety of organic contaminants, such as S-metolachlor (log KD = 3.25 ± 0.12). During atmospheric transport, soot can be chemically transformed by reactive oxygen species including NO2. The presence of natural organic matter (NOM) in surface waters can further affect the aquatic fate of soot. To better understand the processes driving the fate of soot and its interactions with contaminants, pristine and NO2-transformed model soot suspensions were investigated in the presence and absence of NOM. NO2-oxidized soot showed a smaller particle size, a higher number of particles remaining in suspension, and a decreased sorption of S-metolachlor (log KD = 2.47 ± 0.40). In agreement with findings for other CNPs, soot stability against aggregation was increased for both pristine and NO2 transformed soot in the presence of NOM.


 Please wait while we load your content...
Please wait while we load your content...