The formation mechanism of chloropicrin from methylamine during chlorination: a DFT study†
Abstract
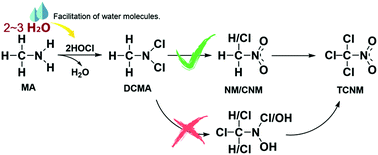
Chloropicrin (TCNM) as one of the most frequently detected nitrogenous disinfection byproducts (N-DBPs) has attracted extensive attention due to its high toxicity. Although much research work on TCNM has been done, its formation mechanism during chlorination has not been known clearly yet. In this study, TCNM formation mechanisms from methylamine (MA) during chlorination, including N-chlorination of MA by hypochlorous acid to generate dichloromethylamine (DCMA) first and then oxidation of DCMA to form nitromethane (NM) and chloronitromethane (CNM), and finally TCNM formation from C-chlorination of NM and CNM, were investigated by using the DFT method. The calculated results show that in N-chlorination of MA, 2–3 water molecules involved in the reaction facilitate Cl+ and proton transfer with the activation free energies (ΔG≠) for the first and second chlorination in the range of 4–7 and 14–17 kcal mol−1, respectively, which are in good agreement with the experimental results. Formation of NM and CNM proceeds through a series of elimination, addition, and oxidation reactions with ΔG≠ of the rate-limiting steps being around 34–37 kcal mol−1, and the subsequent C-chlorination of methyl in NM and CNM by hypochlorous acid is a rapid process with ΔG≠ below 7 kcal mol−1. This infers that the TCNM formation mechanism from DCMA is more likely to undergo first N-oxidation and then C-chlorination. These results can explain the experimental findings that the molar yield of TCNM from MA during chlorination is low (<0.1%) whereas that from NM is rather high (∼45%). This work will be helpful to elucidate formation mechanisms of all the halonitromethanes during chlorination.


 Please wait while we load your content...
Please wait while we load your content...