Mutually-dependent kinetics and energetics of photocatalyst/co-catalyst/two-redox liquid junctions†
Abstract
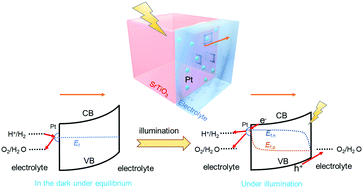
Water-splitting by photocatalyst particles has attracted much attention recently for its potential to produce renewable H2 at scale. However, the correlation between the energetics at photocatalyst/co-catalyst/water interfaces and their interfacial charge-transfer kinetics is still elusive, especially when the energetics are expected to vary spatially along the liquid-junction interface. First, we derived a kinetic model for photocatalyst particles in contact with two-redox potentials, H+/H2 and O2/H2O, i.e., a semiconductor/two-redox liquid junction. We adopted the principle of detailed balance proven for one-redox liquid junctions and extended this principle to a locally out-of-equilibrium electrolyte containing multiple redox potentials, the condition typical for photocatalysts. To validate the model, we established a characterization framework to simulate photocatalyst operation by using photoelectrodes. The open-circuit conditions mimicked operating photocatalyst surfaces; and the (quasi-) Fermi levels, probed by ohmic back contacts, indicated charge-separation efficiency. Quantitative data fitting further validated the two-redox kinetic model. These characterizations correlated local energetics with multi-electron charge-transfer kinetics, which exhibit tuneable branching ratios controlled by H2-and-O2 gas-mixture compositions and co-catalyst selectivity. Unlike the conventional photoelectrode/electrolyte interfaces, SrTiO3 model particles decorated with Pt co-catalysts were found to bear liquid-junction interfaces of spatially varying energetics with designated reductive and oxidative sites. It is shown that, uniquely for photocatalysts, the local kinetic-controlled energetics vary spatially across photocatalyst/co-catalyst/water interfaces of individual particles, and affect charge-separation efficiency sensitively. The mutually dependent behaviour between local kinetics and spatially varying energetics were confirmed for two practical photocatalytic systems, Al-doped SrTiO3 and Ta3N5. This study exemplified and elucidated the design principles for developing efficient photocatalysts.
- This article is part of the themed collection: Energy & Environmental Science Cover Art


 Please wait while we load your content...
Please wait while we load your content...