FSI-inspired solvent and “full fluorosulfonyl” electrolyte for 4 V class lithium-metal batteries†
Abstract
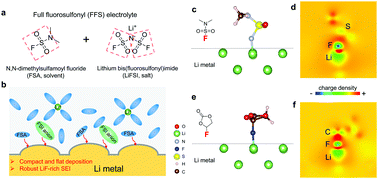
High-voltage rechargeable lithium-metal batteries (LMBs) require electrolytes that are compatible with both the Li metal anode (LMA) and the metal-oxide cathode. Herein, by imitating the fluorosulfonyl imide group from a well-known LMA-compatible salt, lithium bis(fluorosulfonyl) imide (LiFSI), we come up with an organic solvent dimethylsulfamoyl fluoride (FSO2NC2H6), a fluorosulfonamide (FSA) with two methyl substituents, to develop a new “full fluorosulfonyl” (FFS) electrolyte. Remarkably, it enables a highly reversible LMA with an excellent initial coulombic efficiency (CE) ∼91%, and rapidly approaching 99% within only 10 cycles, with average CE outperforming the well-known LMA-compatible fluoroethylene carbonate (FEC)-based electrolyte. Furthermore, benefitting from its high anodic stability against the oxidative LiNi0.6Mn0.2Co0.2O2 (NMC622) and LiMn2O4 (LMO) surfaces, the Li‖NMC622 cell retains 89% of its original capacity after 200 cycles using a limited Li excess anode. This electrolyte design strategy opens a new avenue for exploring new medium-concentration organic electrolytes for 4 V class lithium-metal batteries (LMBs).


 Please wait while we load your content...
Please wait while we load your content...