Operando X-ray characterization of high surface area iridium oxides to decouple their activity losses for the oxygen evolution reaction†
Abstract
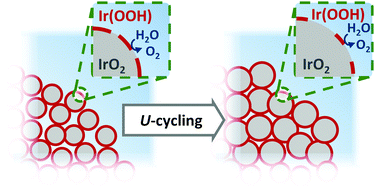
IrO2 is the state-of-art O2-evolution reaction (OER) electrocatalyst implemented in proton exchange membrane electrolyzers, but in the near future iridium's ultra-low availability could threaten the successful development of this technology. To minimize this dependency, Ir-oxides with enhanced mass-specific surface areas and OER-activities are progressively being developed, but often suffer from a poorly understood deactivation under operating conditions. To understand this activity loss, in this study we used a modified Adams’ fusion method to produce an Ir-oxide with a surface area of ≈350 m2 g−1 that consists of nano-disks with their surface partially covered by a layer of Ir(OOH). In order to investigate the effect of this surface oxidation state on the catalyst's reactivity and stability, a fraction of this as-synthetized sample was submitted to a second heat-treatment in air to further oxidize its surface (i.e., yielding IrO2 with ≈250 m2 g−1). While electrochemical characterization through rotating disk electrode voltammetry unveiled that the as-synthesized catalyst features a ≈2-fold larger surface-specific OER-activity than its heat-treated derivative, it also undergoes a greater loss of such activity in the course of an accelerated stress test (AST) that mimics electrolyzer startup/shutdown (≈45 vs. ≈30% OER-current decrease for the as-synthetized sample vs. its heat-treated derivative, respectively). Since ex situ analyses (e.g., through X-ray photoelectron spectroscopy) were not sufficient to explain this difference in stability, the operando changes in the samples' morphology and chemical composition were assessed using a recently developed apparatus that combines small angle X-ray scattering (SAXS) and X-ray absorption spectroscopy (XAS). While the XAS measurements demonstrated the compositional stability of both catalysts (i.e., oxidation state and local geometric structure), SAXS showed that the as-synthetized catalyst is made of two-dimensionally agglomerated disks that become thinner and wider in the course of the AST, whereas the heat-treated sample is composed of morphologically stable, sintered particles in the form of rough and porous agglomerates. Considering the complementary information provided by these operando and ex situ techniques, it was then possible to quantify the contributions of Ir-dissolution, surface area loss and changes in the surface oxidation state to the destabilization of both catalysts.


 Please wait while we load your content...
Please wait while we load your content...