Towards membrane-electrode assembly systems for CO2 reduction: a modeling study†
Abstract
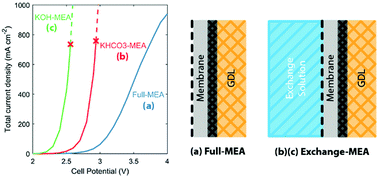
Membrane-electrode assemblies (MEAs) are an attractive cell design for the electrochemical reduction of CO2 because they exhibit low ohmic loss and high energy efficiency. We describe here the development and application of a multiphysics model to investigate the fundamental limitations of two MEA designs: one with gaseous feeds at both the anode and cathode (full-MEA), and the other with an aqueous anode feed (KHCO3 or KOH exchange solution) and a gaseous cathode feed (exchange-MEA). The total current density for the three cases follows the order: KOH-MEA > KHCO3-MEA > full-MEA. This trend is established by examining the distribution of the applied voltage. We show that the main charge-carrying species are carbonate anions for an MEA that uses an anion-exchange membrane (AEM). The amount of CO2 consumed but not converted to CO decreases with increasing current densities above 100 mA cm−2 for a full-MEA, but converges to 50% for exchange-MEAs. The full-MEA becomes limited by ohmic resistance as the membrane dehydrates with increasing cell temperature, and eventually becomes limited due to water mass transport. The exchange-MEAs can maintain membrane hydration and the local ion concentration at the anode, but are limited by salt precipitation at the cathode, as well as a higher tendency to flood. Finally, we explore the effects of temperature and discuss the possibility of increasing water supply to the full-MEA to improve its performance at elevated temperatures. The MEA model and the understanding of MEA performance for the electrochemical reduction of CO2 presented in this study should help guide the design of next-generation CO2 reduction cells.


 Please wait while we load your content...
Please wait while we load your content...