On the energetic efficiency of producing polyoxymethylene dimethyl ethers from CO2 using electrical energy†
Abstract
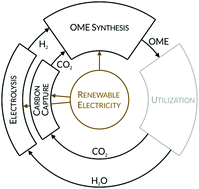
Polyoxymethylene dimethyl ethers (OME) are promising electricity-based fuels (e-fuels). They enable low emissions of particulate matter and nitrous oxides. In this work, the energetic efficiency of OME production from CO2 employing electrical energy is calculated. CO2 capture is included in the analysis by considering direct air capture and post-combustion capture technologies. CO2 is converted to methanol using hydrogen produced by electrolysis. Two routes for OME synthesis are studied: OME synthesis directly from methanol and formaldehyde and OME synthesis via the two intermediates methylal and trioxane. The energetic efficiency of OME production is evaluated for different levels of heat integration and for variable electrolysis efficiencies. For an electrolysis efficiency of 60%, it ranks between 24.3 and 36.7%. The production of 1 kg OME3–5 (LHV of 18.9 MJ per kg) has an energy demand of 51.6–78.0 MJ. A comparison of the energetic efficiencies of the production of different e-fuels and the evaluation of future potential sets OME in an overall context.


 Please wait while we load your content...
Please wait while we load your content...