Controlling dinitrogen functionalization at rhenium through alkali metal ion pairing†
Abstract
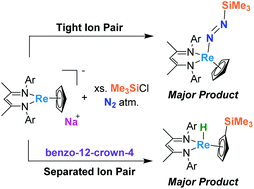
The rhenium(I) salt Na[Re(η5-Cp)(BDI)] can be cooled in solution under a dinitrogen atmosphere to selectively access complexes containing rhenium(III) centers bound to direduced, doubly-bonded N2 (i.e. diazenide) fragments. We demonstrate this reactivity is critically dependent on ion pairing involving the Na+ ion in the starting material, as N2 binding by Na[Re(η5-Cp)(BDI)] proved to be much less favorable when the Na+ was sequestered by benzo-12-crown-4. The analogous chemistry of Na[Re(η5-Cp)(BDI)] with carbon monoxide (CO) and 2,6-xylylisocyanide (XylNC) was also investigated, which provided structural and spectroscopic bases for determining the impact of ion pairing on π-acid activation in this system.


 Please wait while we load your content...
Please wait while we load your content...
