Photophysical properties of some novel tetraphenylimidazole derived BODIPY based fluorescent molecular rotors†
Abstract
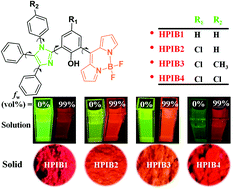
The strategic design, synthesis and thorough characterization of four novel hydroxyl-substituted tetraphenylimidazole (HPI) based boron dipyrromethene (BODIPY) fluorophores (HPIB1–HPIB4) have been reported. Single crystal X-ray structure determination unveiled non-planar twisted orientations for these molecules. The non-planar orientations entirely restrict detrimental π–π interactions and avoid the non-radiative relaxation pathway for excited states in the solid/aggregated state and make them AIE active. The AIE characteristics of these compounds have been related to fine J-aggregation (evident from their crystal structures) along with restricted intra-molecular rotations (RIRs). These compounds display significant sensitivity toward viscosity and can serve as fluorescent molecular rotors due to multiple phenyl groups around the imidazole ring, which has been confirmed by measuring fluorescence quantum yields and lifetimes.


 Please wait while we load your content...
Please wait while we load your content...