Design, synthesis, characterization of peripherally tetra-pyridine-triazole-substituted phthalocyanines and their inhibitory effects on cholinesterases (AChE/BChE) and carbonic anhydrases (hCA I, II and IX)†
Abstract
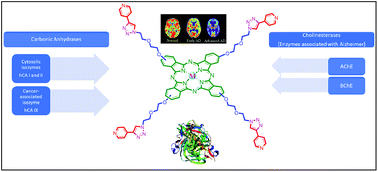
In this study, phthalocyanine precursors (5 and 9) and 1,2,3-triazole-substituted metal-free and metallo phthalocyanines (9a–c) were designed and synthesized for the first time and evaluated in vitro for key molecular targets. The structures of the novel compounds were characterized via FT-IR, 1H/13C NMR, UV-Vis, and mass spectroscopy. The inhibitory activities of the compounds were tested against human carbonic anhydrase isoforms hCA I, II (cytosolic, ubiquitous isozymes), and IX (transmembrane, cancer-associated isozyme) and cholinesterases (AChE and BChE, which are associated with Alzheimer's disease). Among the three phthalocyanines and starting compounds, 9b showed the most interesting profile as a nanomolar selective inhibitor of hCA I (Ki = 37.2 nM) and 9c showed the most effective inhibitory effect on hCA II, IX, AChE and BChE (Ki = 41.9, 27.4, 5.8 and 45.8 nM, respectively). This study is also the first example of cancer-associated isozyme hCA IX inhibition by phthalocyanines.


 Please wait while we load your content...
Please wait while we load your content...