Promoting effects of water on the NH3-SCR reaction over Cu-SAPO-34 catalysts: transient and permanent influences on Cu species†
Abstract
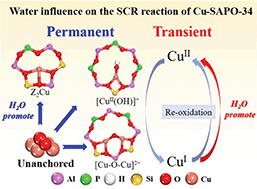
Cu-SAPO-34 catalysts with varied Cu loadings were synthesized through ion exchange to study the influence of water on the NH3-SCR reaction. The catalytic activities were evaluated by selective catalytic reduction of NO under a reactant feed in the presence/absence of water. Transient experiments were designed to study the response of NO conversion to the presence of water. H2-TPR and DFT calculations were performed to study the reducibility of Cu species. NH3-TPD and XPS were conducted to reveal the migration of Cu species. The results show that water could remarkably improve NO reduction activities and the promoting effect is more significant on the catalyst with low Cu loading. Both transient and permanent influences were found in this promoting phenomenon. For the transient influence, water has been proved to accelerate the re-oxidation half-cycle. Moreover, water can enhance the promoting effect of the SCR feed on the migration of Cu species. These unanchored Cu ions migrate to defect sites to form active sites, which lead to a permanent influence of water.


 Please wait while we load your content...
Please wait while we load your content...