Copper complexes for the promotion of iminopyridine ligands derived from β-alanine and self-aldol additions: relaxivity and cytotoxic properties†
Abstract
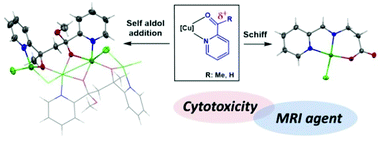
In the study presented herein, we explore the ability of copper complexes with coordinated pyridine-2-carboxaldehyde (pyca) or 2-acetylpyridine (acepy) ligands to promote the addition of amines (Schiff condensation) and other nucleophiles such as alcohols (hemiacetal formation). Distinct reactivity patterns are observed: unlike pyca complexes, acepy copper complexes can promote self-aldol addition. The introduction of a flexible chain via Schiff condensation with β-alanine allows the possibility of chelate ring ring-opening processes mediated by pH. Further derivatization of the complex [CuCl(py-2-C(H)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NCH2CH2COO)] is possible by replacing its chloride ligand with different pseudohalogens (N3−, NCO− and NCS−). In addition to the change in their magnetism, which correlates with their solid-state structures, more unexpected effects in their cytotoxicity and relaxitivities are observed, which determines their possibility to be used as MRI contrast agents. The replacement of a chloride by another pseudohalogen, although a simple strategy, can be used to critically change the cytotoxicity of the Schiff base copper(II) complex and its selectivity towards specific cell lines.
NCH2CH2COO)] is possible by replacing its chloride ligand with different pseudohalogens (N3−, NCO− and NCS−). In addition to the change in their magnetism, which correlates with their solid-state structures, more unexpected effects in their cytotoxicity and relaxitivities are observed, which determines their possibility to be used as MRI contrast agents. The replacement of a chloride by another pseudohalogen, although a simple strategy, can be used to critically change the cytotoxicity of the Schiff base copper(II) complex and its selectivity towards specific cell lines.


 Please wait while we load your content...
Please wait while we load your content...