MLi2Ti6O14 (M = Sr, Ba, and Pb): new cathode materials for magnesium–lithium hybrid batteries†
Abstract
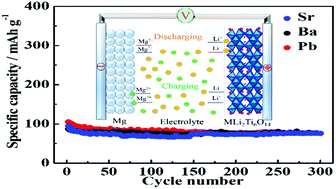
Magnesium–lithium hybrid batteries (MLHBs) are playing an increasingly important role in energy storage systems owing to their abundant raw materials and favorable safety characteristics. Consequently, MLi2Ti6O14 (M = Sr, Ba, and Pb) compounds have been synthesized via a sol–gel method, followed by calcination. For the first time, as cathodes for MLHBs, MLi2Ti6O14 (M = Sr, Ba, and Pb) showed good electrochemical properties. For example, at 50 mA g−1, the specific capacities of MLi2Ti6O14 (M = Sr, Ba, and Pb) after 300 cycles are 75.6, 68.2 and 76.3 mA h g−1, respectively. In addition, MLi2Ti6O14 (M = Sr, Ba, and Pb) also possess outstanding rate performances. Importantly, the ion storage mechanism of MLi2Ti6O14 (M = Sr, Ba, and Pb) compounds in MLHBs was studied with PbLi2Ti6O14 as the representative. These results reveal that MLi2Ti6O14 (M = Sr, Ba, and Pb) have good electrochemical reversibility, and can be used as cathodes for MLHBs.


 Please wait while we load your content...
Please wait while we load your content...