Substituents lead to differences in the formation of two different butterfly-shaped Ni II2Dy III2 clusters: structures and multistep assembly mechanisms†
Abstract
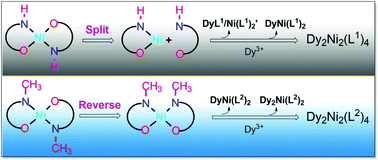
The most effective way to understand reaction mechanisms and kinetics is to identify the reaction intermediates and determine the possible reaction patterns. The influencing factors that must be considered in the self-assembly of clusters are the type of ligand, metal ion, coordination anion and the pH of the solution. However, changes in ligand substituents resulting in different self-assembly processes to obtain different types of structures are still very rare, especially with –H and –CH3 substituents, which do not exert significant steric hindrance effects. In this study, planar mononuclear Ni(L1)2 (L1 = 2-ethoxy-6-(iminomethyl)phenol) was dissolved in methanol and combined with Dy(NO3)3·6H2O for 48 h at room temperature to obtain a butterfly-like Ni2Dy2 cluster ([Dy2Ni2(L1)4(CH3O)2(NO3)4], 1). The Dy(III) ions in cluster 1 are in an O8N coordination environment, and the Ni(II) ions are in an O5N coordination environment. High-resolution electrospray ionization mass spectrometry (HRESI-MS) was used to track species changes during the formation of cluster 1. Six key intermediate fragments were screened, and the self-assembly mechanism was proposed as Ni(L1)2 → HL1 + NiL1 → DyL1/Ni(L1)2′ → DyNi(L1)2 → Dy2Ni2(L1)4. Through this assembly mechanism, we found that Ni(L1)2 was first cleaved into HL1 + NiL1 and then further assembled to obtain 1. Another butterfly-like tetranuclear heterometallic cluster ([Dy2Ni2(L2)4(CH3O)2(NO3)4], 2) was obtained using planar mononuclear Ni(L2)2 (L2 = (E)-2-ethoxy-6-((methylimino)methyl)phenol) with –CH3 substitution on the nitrogen atom under the same reaction conditions. The structural analysis of cluster 2 showed that the Dy(III) ions are in an O9 coordination environment, and the Ni(II) ions are in an O4N2 coordination environment. HRESI-MS was used to trace species changes during the formation of 2, and the assembly mechanism was proposed as Ni(L2)2 → DyNi(L2)2 → Dy2Ni(L2)2 → Dy2Ni2(L2)4. Analysis of the assembly mechanism of 2 showed that Ni(L2)2 was twisted during the reaction, and its coordination point was exposed to capture the Dy(III) ions. Finally, Dy(NO3)3·6H2O was replaced with NaN3 to obtain a [Ni2Na2(L2)4(N3)4] cluster (3) under the same reaction conditions and verify the above-mentioned torsion step. HRESI-MS was also used to trace the assembly process, and the assembly mechanism was proposed as Ni(L2)2 → NiNa(L2)2 → NiNa2(L2)2 → Ni2Na2(L2)4. Herein, the effect of interference from substitution and the regulation self-assembly process were discovered in the formation of 3d–4f heterometallic clusters, and different types of coordination clusters were obtained.


 Please wait while we load your content...
Please wait while we load your content...