Deoxydehydration of polyols catalyzed by a molybdenum dioxo-complex supported by a dianionic ONO pincer ligand†
Abstract
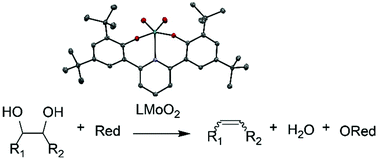
Deoxydehydration (DODH) is the net reduction of diols and polyols to alkenes or dienes and water. Molybdenum cis-dioxo bis-phenolate ONO complexes were synthesized and have been shown to be active for DODH. Catalysts were screened for activity at 150–190 °C, and appreciable yields of up to 59% were obtained. PPh3, Na2SO3, Zn, C, 3-octanol and 2-propanol were screened as reductants. Additionally, the reactivities of a variety of diols were screened. With (R,R)-(+)-hydrobenzoin as substrate, DODH occurs via a mechanism where reduction of the Mo catalyst is a result of diol oxidation to form two equivalents of aldehyde. These reactions result in complete conversion and near quantitative yields of trans-stilbene and benzaldehyde.


 Please wait while we load your content...
Please wait while we load your content...