Facile synthesis and electrochemical Mg-storage performance of Sb2Se3 nanowires and Bi2Se3 nanosheets†
Abstract
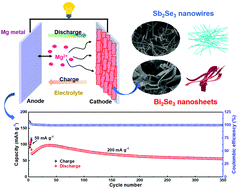
Rechargeable Mg batteries are considered as low-cost and reliable candidates for efficient energy storage, but their development is blocked by the lack of suitable cathode materials. In this work, Sb2Se3 nanowires and Bi2Se3 nanosheets are fabricated by facile one-step hydrothermal methods and their Mg-storage performances are systematically investigated. The results show that the Bi2Se3 nanosheets with stable hierarchical 2D structure exhibit a better performance. Because of its thin nanosheet structure, Bi2Se3 provides a high Mg-storage capacity of 144 mA h g−1 and a remarkable rate capability with 65 mA h g−1 delivered at 1000 mA g−1. Bi2Se3 also exhibits an outstanding cyclability over 350 cycles owing to its hierarchical structure. Furthermore, this study reveals that the electrochemical charge/discharge cycling is a typical conversion reaction occurring between Bi3+ and metallic Bi0. Kinetic investigation suggests that the high performance of Bi2Se3 is attributed to both its intrinsic nature and its thin nanosheet structure facilitating solid-state Mg2+ diffusion. The present work highlights the selection principle of conversion cathodes for rechargeable Mg batteries, namely matching a soft anion with a quasi-soft metal cation. Moreover, the facile synthesis approach is also used for low-dimensional main-group VI metal chalcogenides to improve the Mg-storage performance.


 Please wait while we load your content...
Please wait while we load your content...