Irreversible adsorption of acidic, basic, and water gas molecules on calcium-deficient hydroxyapatite†
Abstract
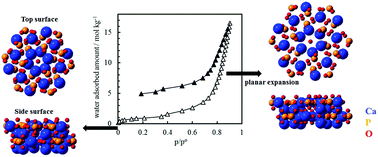
Hydroxyapatite [Ca10(PO4)6(OH)2, HAP] has P–OH Brønsted acidic sites, Ca2+ Lewis acidic sites, and OH− and O2− basic sites on which acidic and basic gas molecules can be selectively adsorbed, and has no micropore onto which various molecules adsorb regardless of the chemical properties of gas molecules. The interaction between the surface sites and acidic and basic gas and water molecules has been investigated by evaluating the adsorption properties of various molecules on the surfaces of calcium-deficient HAP. The specific adsorption sites were assessed by examining the reversible and irreversible adsorption of NH3, CO2, aldehydes, and water vapor on HAP at the temperature of 298 K, using two HAP samples with different Ca/P ratios, but similar structures and surface areas: Ca-deficient HAP with an extreme lower Ca/P ratio (named P-HAP) and one with a higher Ca/P ratio (named C-HAP). Irreversible adsorption of NH3 on C-HAP is attributed to the adsorption on both Ca2+ Lewis acidic and P–OH Brønsted acidic sites. Irreversible adsorption on P-HAP is attributed to the adsorption on P–OH Brønsted acidic sites only. Irreversible adsorption of CO2 occurred on C-HAP only, and preferentially on OH− basic sites. Acetaldehyde undergoes a catalytic reaction over both OH− basic sites and surface P–OH Brønsted acidic sites at 298 K. Water irreversible adsorption was extensively observed for P-HAP, and water was barely desorbed at low pressures. In situ powder X-ray diffraction showed an asymmetric expansion of the lattice in the [100] direction, indicating that water was incorporated into P-HAP crystals, especially on structural OH− sites. Irreversible adsorption of acidic and basic molecules was therefore less observed on P-HAP than on C-HAP, but P-HAP had considerable irreversible adsorption of water vapor with associated asymmetric lattice expansion. The incorporation of water vapor was first observed and could be useful to improve adsorption or catalytic performance with the mediation of water vapor and/or hydration.


 Please wait while we load your content...
Please wait while we load your content...