Neutral and cationic phosphine and arsine complexes of tin(iv) halides: synthesis, properties, structures and anion influence†
Abstract
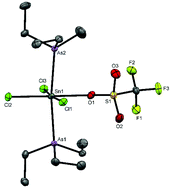
The reaction of trans-[SnCl4(PR3)2] (R = Me or Et) with trimethylsilyltriflate (TMSOTf) in CH2Cl2 solution substitutes one chloride to form [SnCl3(PR3)2(OTf)]; addition of excess TMSOTf does not substitute further chlorides. The complexes have been fully characterised by microanalysis, IR and multinuclear NMR (1H, 13C{1H}, 19F{1H}, 31P{1H}, 119Sn) spectroscopy. The crystal structure of [SnCl3(PMe3)2(OTf)] revealed mer-chlorines and trans-phosphines. In contrast, trans-[SnBr4(PR3)2], [SnCl4{Et2P(CH2)2PEt2}], [SnCl4{o-C6H4(PMe2)2}] and [SnCl4{o-C6H4(AsMe2)2}] did not react with TMSOTf in CH2Cl2 solution even after 3 days. The arsine complexes, [SnX4(AsEt3)2] (X = Cl, Br), were confirmed as trans-isomers by similar spectroscopic and structural studies, while attempts to isolate [SnI4(AsEt3)2] were unsuccessful and reaction of SnX4 with SbR3 (R = Et, iPr) resulted in reduction to SnX2 and formation of R3SbX2. trans-[SnCl4(AsEt3)2] is converted by TMSOTf into [SnCl3(AsEt3)2(OTf)], whose X-ray structure reveals the same geometry found in the phosphine analogues, with the triflate coordinated. The salts, [SnCl3(PEt3)2][AlCl4] and [SnCl2(PEt3)2][AlCl4]2 were made by treatment of [SnCl4(PEt3)2] with one and two mol. equivalents, respectively, of AlCl3 in anhydrous CH2Cl2, whereas reaction of [SnCl4(AsEt3)2] with AlCl3 produced a mixture including Et3AsCl2 and [Et3AsCl][Sn(AsEt3)Cl5] (the latter identified crystallographically). In contrast, using Na[BArF] (BArF = [B{3,5-(CF3)2C6H3}4]−) produced [SnCl3(PEt3)2][BArF] and also allowed clean isolation of the arsine analogue, [SnCl3(AsEt3)2][BArF]. [SnCl4{o-C6H4(PMe2)2}] also reacts with AlCl3 in CH2Cl2 to form [SnCl3{o-C6H4(PMe2)2}][AlCl4] and [SnCl2{o-C6H4(PMe2)2}][AlCl4]2. Multinuclear NMR spectroscopy on the [AlCl4]− salts show that δ31P and δ119Sn move progressively to high frequency on conversion from the neutral complex to the mono- and the di-cations, whilst 1J(119Sn–31P) follow the trend: [SnCl3{o-C6H4(PMe2)2}]+ > [SnCl4{o-C6H4(PMe2)2}] > [SnCl2{o-C6H4(PMe2)2}]2+. DFT studies on selected complexes show only small changes in ligand geometries and bond lengths between the halide and triflate complexes, consistent with the X-ray crystallographic data reported and the HOMO and LUMO energies are relatively unperturbed upon the introduction of (coordinated) triflate, whereas the energies of both are ca. 4 eV lower in the cationic species and reveal significant hybridisation across the pnictine ligands.
- This article is part of the themed collection: Inorganic chemistry of the p-block elements


 Please wait while we load your content...
Please wait while we load your content...