Abstract
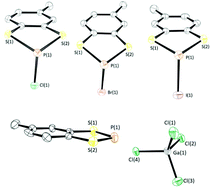
The reaction of either toluene-3,4-dithiol or benzene dithiol with phosphorus(III) trihalides generates the corresponding benzo-fused 1,3,2-dithiaphospholes, RC6H3S2PX (R = Me (1), R = H (2); X = Cl, Br, I). The P-chloro-dithiaphospholes undergo: (a) halogen abstraction reactions with Lewis acids forming phosphenium cations; (b) substitution with LiHMDS base and; (c) reduction chemistry with sodium metal to generate the P–P σ-bonded dimer, (RC6H3S2P)2. Reduction catalysis of aldehydes with pinacolborane using dithiaphospholes is compared with their dioxaphosphole and diazaphosphole counterparts as pre-catalysts, revealing interesting differences in the reactivity of this series of compounds.


 Please wait while we load your content...
Please wait while we load your content...