Ionic liquids with polychloride anions as effective oxidants for the dissolution of UO2†
Abstract
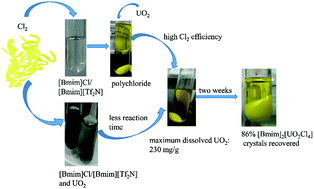
Herein, polychloride ([Cl3]− or/and [Cl5]−) ionic liquids (ILs) were prepared from their imidazolium chloride precursors by the addition of chlorine gas. The highest storage ability of Cl2 was found in the [Bmim]Cl IL among the six imidazolium chlorides [Rmim]Cl (Rmim = Emim, 1-ethyl-3-methylimidazolium; Bmim, 1-butyl-3-methylimidazolium; Pmim, 1-propyl-3-methylimidazolium; Pnmim, 1-pentyl-3-methylimidazolium; Hmim, 1-hexyl-3-methylimidazolium; and Omim, 1-octyl-3-methylimidazolium). When a mixed IL of [Bmim][Tf2N] (1-butyl-3-methylimidazolium bistriflimide) and [Bmim]Cl was used, the IL cation could maintain good stability during the chlorination process, and the imidazolium cation [Bmim]+ could retain almost the same structure after the chlorine gas was introduced into the mixed IL according to 1H NMR spectroscopy. It has been found that the synthesized polychloride ILs not only can successfully dissolve UO2, but can also decrease the consumption of Cl2 and increase the chlorine efficiency. Spectroscopy studies indicate that [UO2Cl4]2− is the principal product in the IL reaction solution. The dissolved uranyl species can be easily recovered from the ILs by crystallization in the form of [Bmim]2[UO2Cl4]. Moreover, even if 57.1 wt% of the lanthanide element, when compared with the total amount of dissolved uranium and lanthanide elements, was dissolved in the IL mixture, only uranium-containing crystals could be isolated from the system.


 Please wait while we load your content...
Please wait while we load your content...