Four new dual-functional electro-catalysts formed from small molybdenum clusters and Cu-pyridyl complexes†
Abstract
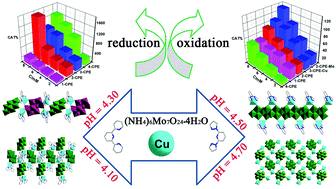
By changing N-heterocyclic ligands in the same Mo7/Cu/N-ligand reaction systems, four new organic–inorganic hybrids based on isopolymolybdates, [Cu2(tpy)2(β-Mo8O26)0.5(γ-Mo8O26)0.5]·0.25H2O (1), [Cu2(tpy)2(H2O)2(β-Mo8O26)] (2), [Cu(bpy)(Mo3O10)]·H2O (3), and [Cu(bpy)(H2O)(β-Mo8O26)0.5]0.5 (4) (tpy = 2,2′:6′,2′′-terpyridine and bpy = 2,6-bis(pyrazol-1-yl) pyridine), were prepared using hydrothermal methods at different pH values. X-ray structural analysis shows that compound 1 has a 1D {-β-[Mo8O26]-Cu2-γ-[Mo8O26]}n straight chain structure with mixed β-[Mo8O26] and γ-[Mo8O26] polyoxoanions; compound 2 possesses a 3D supramolecular structure based on [Cu(tpy)]2+ motifs and β-[Mo8O26] clusters; and compound 3 has a 1D chain structure built from [Cu(bpy)]2+ and [Mo3O10]2− units. In compound 4, [β-Mo8O26] clusters are linked by [Cu(bpy)]2+ motifs to give rise to a 2D sheet structure including {(β-Mo8O26)4Cu4} 8-membered rings. Cyclic voltammograms of compounds 1–4 display discrepant dual-functional electro-catalytic activities toward the reduction of nitrite and the oxidation of ascorbic acid in acidic solution. Electrocatalytic tests indicate that the [Mo3O10]2−-based organic–inorganic hybrid exhibits better electro-catalytic performances than [Mo8O26]4−-type hybrids towards oxidation and reduction.


 Please wait while we load your content...
Please wait while we load your content...