Ferrocenyl palladacycles derived from unsymmetrical pincer-type ligands: evidence of Pd(0) nanoparticle generation during the Suzuki–Miyaura reaction and applications in the direct arylation of thiazoles and isoxazoles†
Abstract
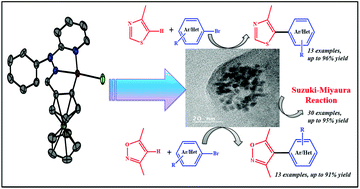
A new family of ferrocenyl-palladacycle complexes Pd(L1)Cl (Pd1) and Pd(L2)Cl (Pd2) were synthesized and characterized by UV-visible, IR, ESI-MS, and NMR spectral studies. The molecular structures of Pd1 and Pd2 were determined by X-ray crystallographic studies. Palladacycle catalyzed Suzuki–Miyaura cross-coupling reactions were investigated utilizing the derivatives of phenylboronic acids and substituted chlorobenzenes. Mechanistic investigation authenticated the generation of Pd(0) nanoparticles during the catalytic cycle and the nanoparticles were characterized by XPS, SEM and TEM analysis. Direct C–H arylation of thiazole and isoxazole derivatives employing these ferrocenyl-palladacycle complexes was examined. The reaction model for the arylation reaction implicating the in situ generation of Pd(0) nanoparticles was proposed.


 Please wait while we load your content...
Please wait while we load your content...