A sulfonated cobalt phthalocyanine/carbon nanotube hybrid as a bifunctional oxygen electrocatalyst†
Abstract
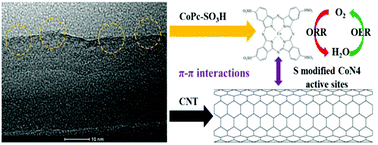
The development of effective bifunctional catalysts for both oxygen reduction reactions (ORRs) and oxygen evolution reactions (OERs) is crucial for improving the performance of charge and discharge processes in rechargeable metal–air batteries. Here, we report a sulfonated cobalt phthalocyanine/carbon nanotube hybrid (CoPc-SO3H/CNT) prepared by a facile anchoring method along with sonication and magnetic stirring. The resulting CoPc-SO3H/CNT hybrid exhibits better catalytic activity for ORRs and OERs than the cobalt phthalocyanine/carbon nanotube hybrid (CoPc/CNT), sulfonated cobalt phthalocyanine (CoPc-SO3H), cobalt phthalocyanine (CoPc) and the carbon nanotube (CNT). The onset potential of CoPc-SO3H/CNT for the ORR in 0.1 M KOH is 0.88 V (vs. RHE), which is higher than that of CoPc/CNT (0.85 V), the CNT (0.80 V), CoPc-SO3H (0.77 V) and CoPc (0.66 V). Meanwhile, the CoPc-SO3H/CNT hybrid shows a much lower OER potential (1.62 V) at a current density of 10 mA cm−2 compared to CoPc-SO3H (1.64 V), CoPc/CNT (1.74 V), the CNT (1.96 V) and CoPc (>2.00 V) in 1 M KOH. Similar patterns are also found in 0.1 M KOH solution. Both the conductive CNT and the electron-withdrawing sulfonic groups are confirmed to benefit the electrochemical oxygen reactions (ORRs/OERs).


 Please wait while we load your content...
Please wait while we load your content...