A water-stable terbium-MOF sensor for the selective, sensitive, and recyclable detection of Al3+ and CO32− ions†
Abstract
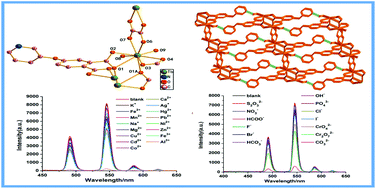
A terbium metal–organic framework associated with mixed 4-(pyridin-3-yloxy)-phthalic acid (H2ppda) and oxalic acid (H2ox) has been synthesized under solvothermal conditions, namely [Tb(ppda)(ox)0.5(H2O)2]n (1). Compound 1 is constructed of 1D binuclear [Tb(ppda)]2 chains and is further connected by ox2− ligands, exhibiting a 2D layered structure. It is worth noting that compound 1 exhibits excellent fluorescence stability over a pH range from 2 to 13 in aqueous solution. Interestingly, compound 1 can detect Al3+ ions with high selectivity and sensitivity among diverse inorganic cations in aqueous solution. In addition, compound 1 is also a highly selective sensing material for CO32− ions without interference from other common anions. Therefore, compound 1 is a promising sensor with a fast response time and low limits of detection for simultaneously detecting Al3+ and CO32− ions, at 5.66 μM for Al3+ ions and 0.38 μM for CO32− ions. Compound 1 displays the lowest limit of detection for CO32− ions using a fluorescent MOF sensor reported to date. The luminescence quenching mechanisms for the different analytes are further explored in detail.


 Please wait while we load your content...
Please wait while we load your content...