Phase transition in the extreme: a cubic-to-triclinic symmetry change in dielectrically switchable cyanide perovskites†
Abstract
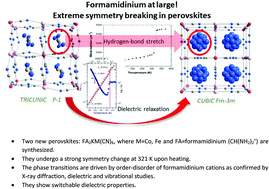
Two novel three-dimensional metal–organic compounds of formula FA2KM(CN)6, where M = Co, Fe and FA = formamidinium (CH(NH2)2+), have been found to crystallize in a perovskite-like architecture. They have been investigated by X-ray diffraction, dielectric and spectroscopic methods as a function of temperature in order to determine the interactions in the crystals and the mechanism of phase transitions occurring at ca. 321 K upon heating. The phase transitions in both crystals are of first order and originate from the ordering of the formamidinium cations below TC. Symmetry reduction (cubic-to-triclinic) seems to be the strongest upon temperature decrease among known metal–organic frameworks. These materials have been proposed as possible switchable dielectrics with a convenient near-room-temperature transition temperature.


 Please wait while we load your content...
Please wait while we load your content...