Synthesis, structure and anion binding properties of 1,8-bis(dimesitylboryl)anthracene and its monoborylated analog†
Abstract
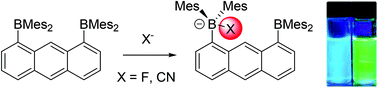
Two boranes, 1-(dimesitylboryl)anthracene (1) and 1,8-bis(dimesitylboryl)anthracene (2), have been synthesized with the spectrophysical properties showing how the inclusion of one or two boron atoms progressively perturbs the π-system of the anthracene backbone. This perturbation is caused by conjugation of the anthracene-π* orbital with the vacant p-orbital on boron. Additionally, both 1 and 2 have a high affinity for fluoride and cyanide anions which are complexed in a 1 : 1 guest–host ratio. The mono-borane 1 is particularly well-suited for cyanide binding, displaying a binding constant of 3 × 107 in THF. Furthermore, as a result of their unique electronic structures, these boranes display a fluorescence response to fluoride anion characterized by a blue shift in the case of 1 and a red shift in the case of 2.


 Please wait while we load your content...
Please wait while we load your content...