Coexistence of Cu(ii) and Cu(i) in Cu ion-doped zeolitic imidazolate frameworks (ZIF-8) for the dehydrogenative coupling of silanes with alcohols†
Abstract
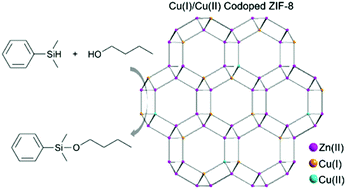
Recently, metal-ion-doped zeolitic imidazolate frameworks have gained considerable attention for their structure tailorability and potential catalytic applications. Herein, Cu ion-doped ZIF-8 nanocrystals were successfully prepared by the mechanical grinding of Cu(NO3)2, ZnO and 2-methylimidazole (HMeIM) using ethanol as an additive. In contrast to the general view that only Cu(II) is present in Cu-doped ZIF-8, we found the coexistence of Cu(II) and Cu(I) in this material, which was supported by XPS and X-ray induced Auger electron spectroscopy (XAES) characterizations. Moreover, ethanol might have acted as a reducer to induce the reduction of Cu(II) during synthesis. Due to the mixed valency of Cu ions, the Cu ion-doped ZIF-8 nanocrystals showed excellent catalytic performance in the dehydrogenative coupling of silanes with alcohols.


 Please wait while we load your content...
Please wait while we load your content...