The diverse mechanisms for the oxidative addition of C–Br bonds to Pd(PR3) and Pd(PR3)2 complexes†
Abstract
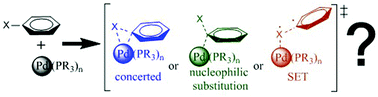
The reaction between bromobenzene and palladium(0) complexes leading to a palladium(II) complex containing bromide and phenyl ligands is studied computationally with DFT methods. Three different mechanisms are considered: concerted, nucleophilic substitution and radical. A systematic analysis is carried out on the effect on each of these mechanisms of a number of variables: the identity of the phosphine (PF3, PH3, PMe3 or PPh3), the nature of the solvent (vacuum, tetrahydrofuran, dimethylformamide or water) and the number of phosphine ligands (mono- or bis-phosphine). The concerted and nucleophilic substitution mechanisms are competitive in many cases, the identity of the preferred one depending on a combination of factors. Additional calculations with bromomethane, bromoethylene and bromoethane are carried out in selected cases for further clarification.
- This article is part of the themed collection: Breaking bonds over many timescales: in celebration of Robin Perutz’s 70th birthday


 Please wait while we load your content...
Please wait while we load your content...