A general benzylic C–H activation and C–C coupling reaction of zirconocenes mediated by C–N bond cleavage in tert-butylisocyanide – unusual formation of iminoacyl complexes†‡
Abstract
Reactions of the zirconocene alkyne complex [rac-(ebthi)Zr(η2-Me3SiC2SiMe3)] (rac-(ebthi) = rac-1,2-ethylene-1,1′-bis(η5-tetrahydroindenyl)) with tert-butylisocyanide and methylbenzenes were investigated. Depending on the stoichiometry, the solvent and the reaction temperature different products were obtained. Starting with the end-on coordination of the isocyanide to the zirconium centre (2), elevated reaction temperatures and an excess of tert-butylisocyanide resulted after the elimination of the alkyne in the formation of zirconocene η2-iminoacyl cyanide complexes 3a–d. These complexes are formed by coupling with a benzyl fragment through C–H bond activation of a methyl group of methylbenzene. The dimeric cyanide bridged zirconocene complex 4 is formed as a side-product of this process. The unexpected dimer and the heterometallacycles were fully characterised, including X-ray crystallography.
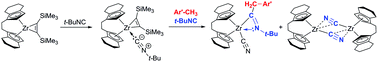
- This article is part of the themed collection: Breaking bonds over many timescales: in celebration of Robin Perutz’s 70th birthday


 Please wait while we load your content...
Please wait while we load your content...