Tuning the reaction pathways of phenanthroline-Schiff bases: routes to novel phenanthroline ligands†
Abstract
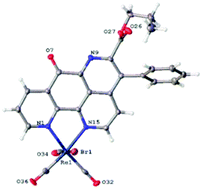
Pyrido-phenanthrolin-7-one compounds are structural analogues of the cytotoxic alkaloid, ascididemin, and would be expected to have interesting biological activities. Synthetic strategies are reported for a novel simple route to form this class of ligand. 1,10-Phenanthrolin-5,6-dione reacts with L-phenylalanine alkyl esters and their para-substituted analogues to form both a phenanthroline–oxazine and a pyrido-phenanthrolin-7-one product. The nature of the major product is dependent on the electronic properties of the para substituent. Successful metal coordination to the pyrido-phenanthrolin-7-one ligand is also presented.
- This article is part of the themed collection: Breaking bonds over many timescales: in celebration of Robin Perutz’s 70th birthday


 Please wait while we load your content...
Please wait while we load your content...