Synthesis, characterisation and electronic properties of naphthalene bridged disilanes†
Abstract
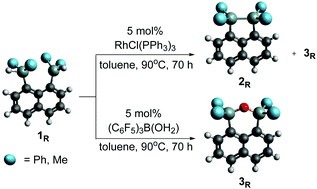
Synthesis of naphthalene bridged disilanes 2R (R = Me, Ph) was performed via catalytic dehydrocoupling. Using RhCl(PPh3)3 as a catalyst, an intramolecular Si–Si bond was readily formed from the corresponding disilyl precursors 1R (R = Me, Ph). For catalytic reactions using (C6F5)3B(OH2), bridged siloxanes (3Ph and 3Me) were observed. Attempts to install the 1,8-naphthalene bridge directly onto a disilane resulted in an unusual product (4), containing two silicon centres bridged through one naphthyl group, and another naphthyl group attached to a single Si centre. In order for this product to form, both a Si to Si hydrogen shift rearrangement as well as Si–Si bond cleavage occurred. The effects of phenyl and methyl substitutions on the structure and electronic properties of the synthesised compounds was investigated by single crystal X-ray diffraction, as well as IR and multinuclear NMR spectroscopic analysis. In addition, theoretical UV-Vis absorption maxima were evaluated using density functional theory (TD-SCF) on a B3LYP/6-31(++)G** level of theory and compared with experimental UV-Vis spectroscopic data.
- This article is part of the themed collection: New Talent: Asia-Pacific


 Please wait while we load your content...
Please wait while we load your content...