A comparative study on the nickel binding ability of peptides containing separate cysteinyl residues†
Abstract
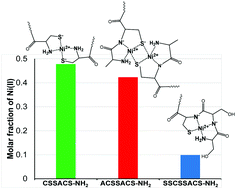
Nickel(II) complexes of peptides CSSACS-NH2, ACSSACS-NH2, SSCSSACS-NH2 and GACAAH-NH2 have been studied by potentiometric and various spectroscopic (UV-vis, CD, NMR, and ESI-MS) techniques. All peptides have high nickel(II) binding ability in the form of square planar complexes and the stability order of the peptides is: CSSACS-NH2 > ACSSACS-NH2 > SSCSSACS-NH2 ∼ GACAAH-NH2. The different metal binding affinities of these peptides are related to the differences in the speciation and in the binding modes of the major species. An almost exclusive formation of bis(ligand) complexes via an (NH2,S−) 5-membered chelate from the amino terminus is characteristic of CSSACS-NH2. The (NH2,N−,S−) tridentate chelate is the major coordination mode of ACSSACS-NH2 but the distant cysteine can also contribute to metal binding. The higher nickel(II) binding ability of A![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) SSA
SSA![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) S-NH2 relative to the peptides containing an N-terminal XY-Cys motif may have important biological consequences. For example, the occurrence of the (NH2,N−,S−,S−) donor set is a common feature of both the ACSSACS-NH2 ligand and the nickel(II) binding loop of the NiSOD enzyme (H
S-NH2 relative to the peptides containing an N-terminal XY-Cys motif may have important biological consequences. For example, the occurrence of the (NH2,N−,S−,S−) donor set is a common feature of both the ACSSACS-NH2 ligand and the nickel(II) binding loop of the NiSOD enzyme (H![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) DLP
DLP![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) G…..,). In the case of SSCSSACS-NH2 and GACAAH-NH2 the amino terminus of one peptide can completely saturate the coordination sphere of the nickel(II) ion via the formation of the (NH2,N−,N−,S−) binding mode. This rules out the formation of bis(ligand) complexes and any contribution of the distant cysteine or histidine to nickel(II) binding in the 1 : 1 complexes. On the other hand the distant cysteine of SSCSSACS-NH2 and histidine of GACAAH-NH2 can behave as independent metal binding sites for the formation of dinuclear complexes in the presence of excess metal ions.
G…..,). In the case of SSCSSACS-NH2 and GACAAH-NH2 the amino terminus of one peptide can completely saturate the coordination sphere of the nickel(II) ion via the formation of the (NH2,N−,N−,S−) binding mode. This rules out the formation of bis(ligand) complexes and any contribution of the distant cysteine or histidine to nickel(II) binding in the 1 : 1 complexes. On the other hand the distant cysteine of SSCSSACS-NH2 and histidine of GACAAH-NH2 can behave as independent metal binding sites for the formation of dinuclear complexes in the presence of excess metal ions.


 Please wait while we load your content...
Please wait while we load your content...