Water adsorption and proton conduction of a cobalt(ii) complex assembled by triazine-based polycarboxylate†
Abstract
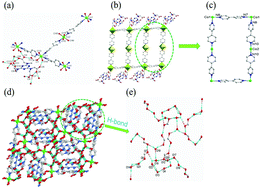
A new flexible triazine-based polycarboxylate coordination polymer, {[Co3(H3TTHA)2(4,4′-bipy)5(H2O)8]·12H2O}n (1), where H6TTHA = 1,3,5-triazine-2,4,6-triamine hexaacetic acid, has been synthesized under hydrothermal conditions and structurally characterized by infrared spectroscopy, elemental analysis, TGA, XRD and X-ray single-crystal diffraction. Structural analysis indicates that 1 displays a planar structure with alternate rectangular structures of 22.695 × 11.485 Å2. The investigation of water vapor adsorption shows that the adsorption capacity of 1 is comparable to that of the typical adsorption material of MCM-41 with 73.86% (41.03 mmol g−1) water uptake at 90% relative humidity (RH). Based on the resistance to water and high-density hydrophilic units as well as abundant hydrogen-bonding networks in the complex, the proton conductivities of 1 under different conditions were measured. The results indicate that the proton conductivity values are highly temperature and relative humidity dependent, with the highest conductivity of nearly 10−3 S cm−1 at 353 K and 98% RH. The Arrhenius activation energy derived in the wide temperature range of 293–353 K is 0.32 eV, corresponding to a typical Grotthuss mechanism.


 Please wait while we load your content...
Please wait while we load your content...