In situ synthesis and electrochemical performance of MoO3−x nanobelts as anode materials for lithium-ion batteries
Abstract
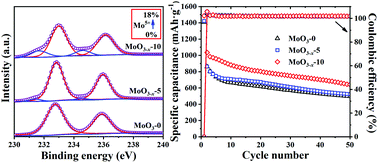
MoO3−x nanobelts with different concentrations of oxygen vacancies were synthesized by a one-step hydrothermal process. XPS test results show that oxygen vacancies are distributed from the exterior to the interior of the MoO3−x nanobelts. As an anode material for lithium-ion batteries, MoO3−x-10 releases excellent rate capacitance. It can maintain a high specific capacitance of about 500 mA h·g−1 at a high current density of 1000 mA·g−1. In the aspect of cycling stability, MoO3−x-10 can retain a high specific capacity of 641 mA h·g−1 after cycling for 50 times at 100 mA·g−1 and 420 mA h·g−1 after cycling for 100 times at 500 mA·g−1. The coexistence of oxygen vacancies and low-valence Mo ions is conducive to the intercalation/de-intercalation of Li ions and to promoting redox reactions. It has been proved to be a significantly effective way in which oxygen vacancies can improve the integrated performance of MoO3−x nanobelts as anode materials.


 Please wait while we load your content...
Please wait while we load your content...