Monitoring fragmentation and oligomerization of a di-μ-methoxo bridged copper(ii) complex: structure, mass spectrometry, magnetism and DFT studies†
Abstract
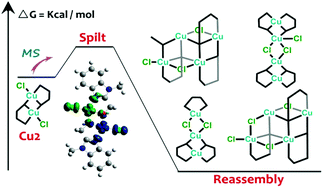
Analyses of the structural information of molecular fragments from the mass spectra of the solid-state products and their reaction solutions allow for the understanding of their formation and of their diverse properties. The reaction of CuCl2 and (1-methyl-1H-benzo[d]imidazole-2-yl)methanol (HL) led only to crystals containing molecular dimers of [Cu2(L)2Cl2] (Cu2). The CuII–CuII distance and Cu–OR–Cu angle in the structure are 3.044 Å and 104.8°, respectively. The magnetic susceptibility (3–400 K) is characterized by a very strong intradimer antiferromagnetic interaction of J = −465 and interdimer zj = −0.83 cm−1. But mass spectrometry of a dissolved single crystal in different source energies identifies both its fragmentation and oligomerization to [CuII3] and [CuII4]. DFT calculations give the relative stabilization energies of the fragments observed in ESI-MS to provide a formation process.


 Please wait while we load your content...
Please wait while we load your content...