Thermodynamic and structural aspects of the aqueous uranium(iv) system – hydrolysis vs. sulfate complexation†
Abstract
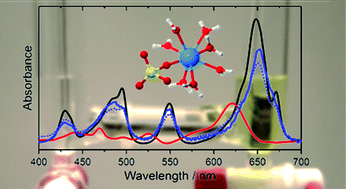
The aquatic species of U(IV) in acidic aqueous solution in the presence of sulfate were studied in the micromolar range by a combined approach of optical spectroscopy (UV/vis and mid-IR), quantum-chemical calculations (QCC), and thermodynamic modelling. The number of species occurring in solution within the pH range 0–2 was assessed by decomposition and fitting of photometric spectra using HypSpec and Geochemist's Workbench software. Single component spectra of U4+, UOH3+, USO42+ and U(SO4)2 were obtained and extinction coefficients ελ were calculated to be 61.7, 19.2, 47.6 and 40.3 L mol−1 cm−1, respectively. Complex formation constants of two U(IV) sulfate species and the first hydrolysis species UOH3+ in infinitely diluted solution were determined by thermodynamic modelling to be log βθ101 = 6.9 ± 0.3, log βθ102 = 11.8 ± 0.5 and log βθ110 = −(0.36 ± 0.1), respectively. No further U(IV) sulfate and hydrolysis species were observed under the prevailing conditions. Molecular structural information of the sulfate species was derived from vibrational spectra and QCC exhibiting a predominant monodentate coordination of the sulfate ions.


 Please wait while we load your content...
Please wait while we load your content...