LiMnPO4-olivine deposited on a nanoporous alloy as an additive-free electrode for lithium ion batteries†
Abstract
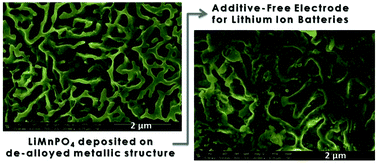
A new strategy for the fabrication of binder- and carbon-free electrodes for lithium ion batteries is demonstrated. The strategy is based on the employment of a nanoporous metallic structure as a mechanically stable and conductive scaffold inside of which an active material is directly grown. The porous metallic structures with the pore size on the nanometric scale were obtained by de-alloying microcrystalline Cu60Ag30Al10, applying the method of selective dissolution of the less noble metals from the alloy. The active material of choice is LiMnPO4-olivine, which displays higher energy density in comparison with the well-known LiFePO4 (701 W h kg−1versus 586 W h kg−1). The improved electrode capacity can be explained by the enhanced diffusion of Li+ into LiMnPO4, achieved by decreasing the size of the phospho-olivine particles, incorporated inside the pores of the metallic structure. This approach enables to us perform the precise engineering of the particle size, which in turn contributes to the improvement of the electrochemical properties of phospho-olivines.


 Please wait while we load your content...
Please wait while we load your content...