Homogeneous vs. heterogeneous catalysis for hydrogen evolution by a nickel(ii) bis(diphosphine) complex†
Abstract
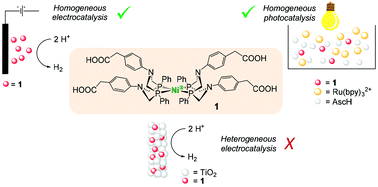
Nickel(II) bis(diphosphine) complex 1 bearing carboxylic acid functional groups at the periphery has been prepared and characterized. Its catalytic ability towards the hydrogen evolution reaction (HER) has been evaluated under different experimental conditions: (i) under homogeneous electrocatalysis in acetonitrile using trifluoroacetic acid as the proton source, (ii) under homogeneous photocatalysis in aqueous solution using Ru(bpy)32+ (where bpy = 2,2′-bipyridine) as the sensitizer and ascorbic acid as the sacrificial electron donor, and (iii) under heterogeneous electrochemical conditions upon grafting onto mesoporous TiO2. The results show that complex 1 is a competent and efficient catalyst for the HER under both homogeneous electro- and photochemical conditions, while undergoes rapid deactivation once attached onto the TiO2 surface. This is attributed to both catalyst binding mode and structural rigidity imparted by covalent grafting, which highlights how catalyst design has to be considered for the direct transposition of molecular catalysis on (photo)electrode surfaces.


 Please wait while we load your content...
Please wait while we load your content...