Synthesis, crystal and electronic structures of Pt-rich phosphides EuPt3P and EuPt6P2†
Abstract
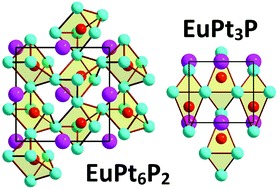
Two new ternary Pt-rich phosphides, EuPt6P2 and EuPt3P, have been prepared via a two-step solid state reaction. Their crystal structures have been determined from powder XRD data. EuPt6P2 is isostructural to SrPt6P2 (cubic, Pa![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , a = 8.4603(1) Å); its crystal structure comprises corner-sharing Pt6P trigonal prisms hosting Eu2+ cations in the cuboctahedral voids of the framework. EuPt3P is isostructural to the SrPt3P anti-perovskite (P4/nmm, a = 5.7452(1) Å and c = 5.4212(1) Å). Magnetization measurements reveal the magnetic response caused by the Eu2+(4f7) cations. EuPt6P2 is paramagnetic exhibiting no phase transitions down to 1.8 K, whereas EuPt3P orders ferromagnetically below 19 K. Similar to SrPt6P2 and SrPt3P, the new compounds are metallic with states near the Fermi level predominantly formed by the 5d orbitals of Pt.
, a = 8.4603(1) Å); its crystal structure comprises corner-sharing Pt6P trigonal prisms hosting Eu2+ cations in the cuboctahedral voids of the framework. EuPt3P is isostructural to the SrPt3P anti-perovskite (P4/nmm, a = 5.7452(1) Å and c = 5.4212(1) Å). Magnetization measurements reveal the magnetic response caused by the Eu2+(4f7) cations. EuPt6P2 is paramagnetic exhibiting no phase transitions down to 1.8 K, whereas EuPt3P orders ferromagnetically below 19 K. Similar to SrPt6P2 and SrPt3P, the new compounds are metallic with states near the Fermi level predominantly formed by the 5d orbitals of Pt.


 Please wait while we load your content...
Please wait while we load your content...