Structure and bonding in reduced boron and aluminium complexes with formazanate ligands†
Abstract
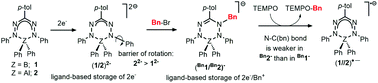
Group 13 complexes of the type [(PhNNC(p-tol)NNPh)ZPh2]2− (Z = B, Al) containing a highly reduced, trianionic formazanate-derived ligand were studied and the differences in the structure, bonding and reactivity between the B and Al compounds were investigated. The increased ionic character in the bonding of the Al complex is evident from the enhanced charge delocalization onto the peripheral ligand substituents (N–Ph) via the π-framework, as shown by the rotation barrier around the N–C(Ph) bond. The electron-rich nature of these compounds allows facile benzylation at the ligand, and the structures of the products were analysed by X-ray crystallography. The products are inorganic analogues of 1-alkylated 1,2,3,4-tetrahydro-1,2,4,5-tetrazines (‘leucoverdazyls’). The six-membered heterocyclic cores of the B and Al compounds are shown to be different, having envelope- and boat-type conformations, respectively. Homolysis of the N–C(benzyl) bond in these compounds was studied by NMR spectroscopy under conditions that trap the organic radical as TEMPO-Bn. Analysis of the reaction kinetics affords activation parameters that approximate the N–C(benzyl) bond strength. The ionic Al compound has one of the weakest N–C bonds reported so far in this type of inorganic leucoverdazyl analogues.
- This article is part of the themed collection: Inorganic chemistry of the p-block elements


 Please wait while we load your content...
Please wait while we load your content...