Fundamental electron-transfer and proton-coupled electron-transfer properties of Ru(iv)-oxo complexes†
Abstract
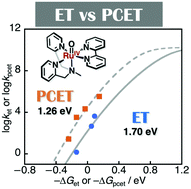
Isolation and characterisation of RuIV(O) complexes were accomplished to investigate their fundamental electron transfer (ET) and proton-coupled ET (PCET) properties. Reorganisation energies (λ) in electron transfer (ET) and proton-coupled ET (PCET) from electron donors to the isolated RuIV(O) complexes have been determined for the first time to be in the range of 1.70–1.88 eV (ET) and 1.20–1.26 eV (PCET). It was suggested that the reduction of the λ values of PCET in comparison with those of ET should be due to the smaller structural change in PCET than that in ET on the basis of DFT calculations on 1 and 1e−-reduced 1 in the absence and presence of TFA, respectively. In addition, the smaller λ values for the RuIV(O) complexes than those reported for FeIV(O) and MnIV(O) complexes should be due to the lack of participation of dσ orbitals in the ET and PCET reactions. This is the first example to evaluate fundamental ET and PCET properties of RuIV(O) complexes leading to further understanding of their reactivity in oxidation reactions.


 Please wait while we load your content...
Please wait while we load your content...
