para metallation of 3-acetyl-chromen-2-one Schiff bases in tetranuclear palladacycles: focus on their biomolecular interaction and in vitro cytotoxicity†
Abstract
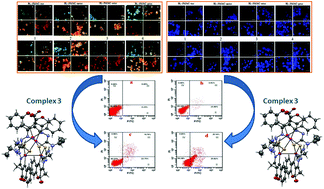
Three tetranuclear (1–3) complexes and a mononuclear (4) palladium(II) complex were synthesized from 3-acetyl-chromen-2-one Schiff base ligands [H2-3MAC-Rtsc] (where R = H [H2-3MAC-tsc]; CH3[H2-3MAC-mtsc]; C2H5[H2-3MAC-etsc] or C6H5[H2-3MAC-ptsc]) and potassium tetrachloropalladate. Their formation was confirmed by spectroscopic techniques and X-ray crystallographic analysis. Their ability to bind with DNA and albumin was analysed by using absorption and emission titrations. The MTT assay was carried out to analyze the anticancer potential of the ligands and synthesized complexes against HepG2 (human liver cancer) and HT-29 (human colon cancer) cells. In addition, the compounds were less toxic when tested against the human normal keratinocyte cells (HaCaT). Ligands and complexes displayed better cytotoxicity with lower IC50 values than the standard drug cisplatin. Further AO-EB and DAPI staining assays were carried out to detect the mode of cell death induced by the complexes i.e. apoptosis or necrosis. The complex 3 showed better cytotoxicity and was further subjected to flow cytometric analysis. The results suggested that the complex 3 induced apoptotic cell death.


 Please wait while we load your content...
Please wait while we load your content...