Syntheses and quadratic nonlinear optical properties of 2,7-fluorenylene- and 1,4-phenylene-functionalized o-carboranes†
Abstract
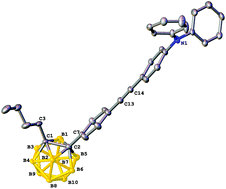
o-Carboranes C-functionalized by (4-substituted-phen-1-yl)ethynyl-1,4-phenyl groups or (2-substituted-fluoren-7-yl)ethynyl-2,7-fluorenyl groups, in which the pendant functionalization is electron-withdrawing nitro or electron-donating diphenylamino groups, have been synthesized and in many cases structurally characterized. Diphenylamino-containing examples coupled via the two π-delocalizable bridges to the electron-accepting o-carborane unit exhibit the greater quadratic optical nonlinearities at 1064 nm (hyper-Rayleigh scattering, ns pulses), the nonlinearities also increasing on proceeding from 1,4-phenylene- to 2,7-fluorenylene-containing bridge. The most NLO-efficient example 2-(n-butyl)-1-(2-((9,9-di(n-butyl)-2-(N,N-diphenylamino)-9H-fluoren-7-yl)ethynyl)-9,9-di(n-butyl)-9H-fluoren-7-yl)-1,2-ortho-carborane, consisting of diphenylamino donor, fluorenyl-containing bridge, o-carborane acceptor, and solubilizing n-butyl units, exhibits large 〈β〉HRS (230 × 10−30 esu) and frequency-independent (two-level model) 〈β0〉 (96 × 10−30 esu) values. Coupling two (2-((9,9-di(n-butyl)-2-(N,N-diphenylamino)-9H-fluoren-7-yl)ethynyl)-9,9-di(n-butyl)-9H-fluoren-7-yl) units to the 1,2-ortho-carborane core affords a di-C-functionalized compound with enhanced nonlinearities (309 × 10−30 esu and 129 × 10−30 esu, respectively).


 Please wait while we load your content...
Please wait while we load your content...