Kinetic stabilization of low-oxidation state and terminal hydrido main group metal complexes by a sterically demanding N,N′-bis(2,6-terphenyl)triazenide†
Abstract
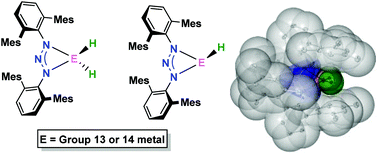
A sterically demanding N,N′-bis(2,6-terphenyl)triazenide was employed to stabilize a number of low-coordinate main group metal terminal hydride complexes. These complexes display enhanced thermal stabilities relative to analogous complexes ligated by β-diketiminates, which we attribute to the steric shielding of the metal hydride moiety by the ligand. We also show this triazenide ligand can stabilize low-oxidation state Group 13 metals. DFT calculations conducted on these triazenide ligated Group 13 metal(I) complexes revealed they possess a narrower HOMO–LUMO gap relative to analogous complexes ligated by other monoanionic N,N′-ligands. In addition, several heteroleptic Group 13 metal(III) and Group 14 metal(II) complexes featuring this triazenide are reported.


 Please wait while we load your content...
Please wait while we load your content...