Synthesis and redox chemistry of Pd(ii) complexes of a pincer verdazyl ligand†
Abstract
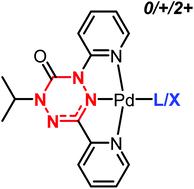
A series of palladium(II) complexes containing a redox-active, tridentate verdazyl ligand of general formula (verdazyl)PdL (L = Cl, CH3CN) are synthesized. The tetrazine core of tridentate verdazyl ligand 5 is flanked by two pyridyl groups, creating a geometry in which the ancillary ligand L is bound trans to the verdazyl ring in the square planar metal complexes. Pd(II) complexes were isolated with the verdazyl ligand in either its neutral radical charge state (6: L = CH3CN, 12: L = Cl) or its closed-shell monoanionic charge state (10: L = CH3CN, 9: L = Cl). The charge state of the ligand was determined using X-ray crystallography and NMR, EPR, and IR spectroscopy. The cyclic voltammograms of radical complexes 6 and 12 each contain a reversible one-electron reduction wave and an irreversible one-electron oxidation wave. The complexes can be chemically interconverted between radical ligand (6, 12) and reduced, closed-shell anion (9, 10) using decamethylferrocene as the reductant and a mixture of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone and fluoroboric acid as the oxidant.


 Please wait while we load your content...
Please wait while we load your content...